Match the hydrocarbons in Column I with the boiling points given in Column II.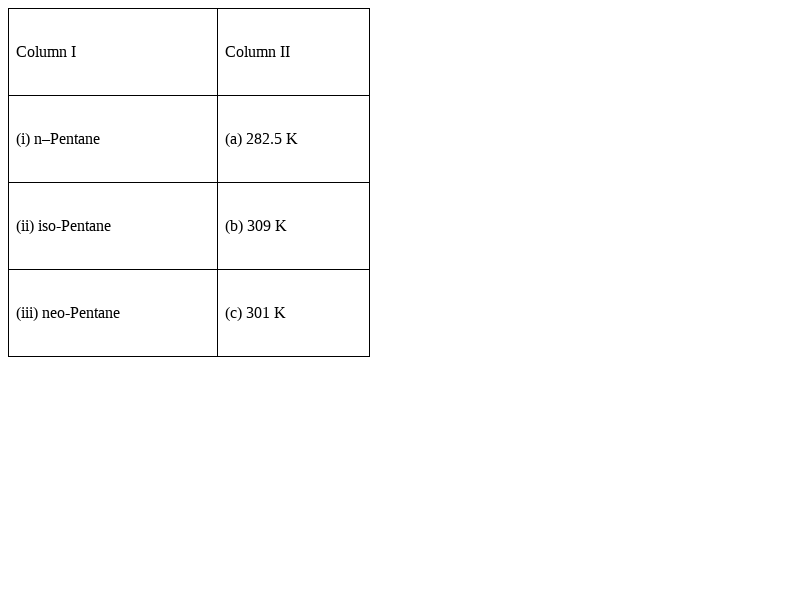
(i)→ (b) , (ii) → (c) , (iii)→(a)
Explanation -
•n-pentane molecules fit closely together; because long chain carbon have greater surface area So, they have strong intermolecular attraction. So boiling is slower than its branched chain isomers.
•Spherical branched chain neopantane exerts little attraction with each other because branching decreases the surface area of the molecule and So, branched hydrocarbon boil easily at low temperature.
•So, neopantane have low boiling point. If branch chain increases boiling point decreases.
1