The oxide that gives H2O2 on treatment with dilute H2SO4 is —
Barium peroxide (BaO2) as we can get from its name, has a per-oxy linkage in it. Due to which its treatment with H2SO4 gives H2O2 . Whereas, the other oxides don’t have the per-oxy linkage due to which hydrogen peroxide cannot be produced.
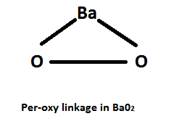
![]()
Whereas, all the other oxides give only water instead of hydrogen peroxide due to the absence of the per-oxy linkage in them.
1