How can production of hydrogen from water gas be increased by using water gas shift reaction?
The water shift gas reaction is used in the formation of synthesis gas (hydrogen and carbon monoxide or carbon dioxide mixture. Here CO is converted to CO2 and H2 is also obtained from water.
Water gas is produced when super-heated steam is passed over red hot coke at very high temperature (around 1300K) in the presence of a Ni catalyst.
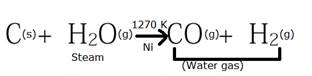
The H2 obtained from this reaction is difficult to obtain due to the difficulty in the removal of poisonous CO gas which is formed along. To fix this difficulty, we oxidise the poisonous CO gas to CO2 gas in another reaction where the products obtained previously is mixed with more steam over heated FeCrO4 catalyst at 673K.
Now, this obtained CO2 gas can be removed easily by passing it through sodium arsenite solution. In this way, production of H2 gas can be increased.
