(i) Draw the gas phase and solid phase structure of H2O2.
(ii) H2O2 is a better oxidising agent than water. Explain.
(i) 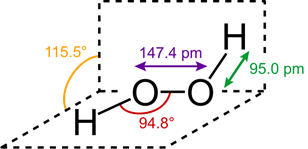
Structure of H2O2 in gas phase
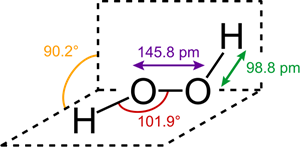
Structure of H2O2 in solid phase
(ii) An oxidising agent is one which oxidises(oxidation number increases) others and itself gets reduced.
The oxidising state of the oxygen atom on H2O2 is -1 which is an intermediate state. It means that it can reduce itself to H2O where oxidation state of O is -2 . So here, H2O2 acts as an oxidising agent. This is not possible for H2O so we can say that H2O2 is a better oxidising agent than water.
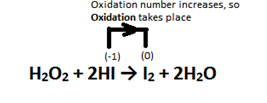
1