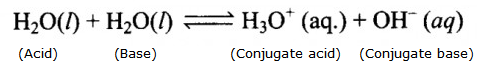When the first element of the periodic table is treated with dioxygen, it gives a compound whose solid state floats on its liquid state. This compound has an ability to act as an acid as well as a base. What products will be formed when this compound undergoes autoionisation?
The first element of the periodic table is hydrogen. When hydrogen reacts with dioxygen, the product formed is water. The solid state of water is ice floats on water due to its lower density. This is because water expands on freezing, so the volume of ice for the same mass of water is more than water. In other words, density of ice is lower than water and hence ice floats on water.
Water has an amphoteric nature which means that it can act both as an acid as well as a base.
Auto protolysis or auto ionisation of water is basically self ionisation of water. It is one of the chemical properties of water. Here, two same molecules react to give ions involving proton transfer.
This reaction also shows the amphoteric nature, which means water can act both as an acid as base.