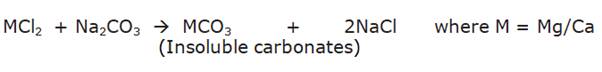In the following questions a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the options given below each question.
Assertion (A) : Permanent hardness of water is removed by treatment with washing soda.
Reason (R) : Washing soda reacts with soluble magnesium and calcium sulphate to form insoluble carbonates.
(i) Statements A and R both are correct and R is the correct explanation of A.
(ii) A is correct but R is not correct.
(iii) A and R both are correct but R is not the correct explanation of A.
(iv) A and R both are false.
(i) Statements A and R both are correct and R is the correct explanation of A.
Permanent hardness of water is due to the presence of soluble salts of magnesium and calcium in the form of chlorides and sulphates in water.
Permanent hardness can be removed by treatment of water with washing soda which contains sodium carbonate. Washing soda reacts with soluble calcium and magnesium chlorides and sulphates in hard water to form insoluble carbonates.