How can D2O be prepared from water? Mention the physical properties in which D2O differs from H2O. Give at least three reactions of D2O showing the exchange of hydrogen with deuterium.
D2O can be prepared by exhaustive or prolonged electrolysis of water or as a by-product in some fertilizer industries.
Some of the physical properties in which D2O differs from H2O are
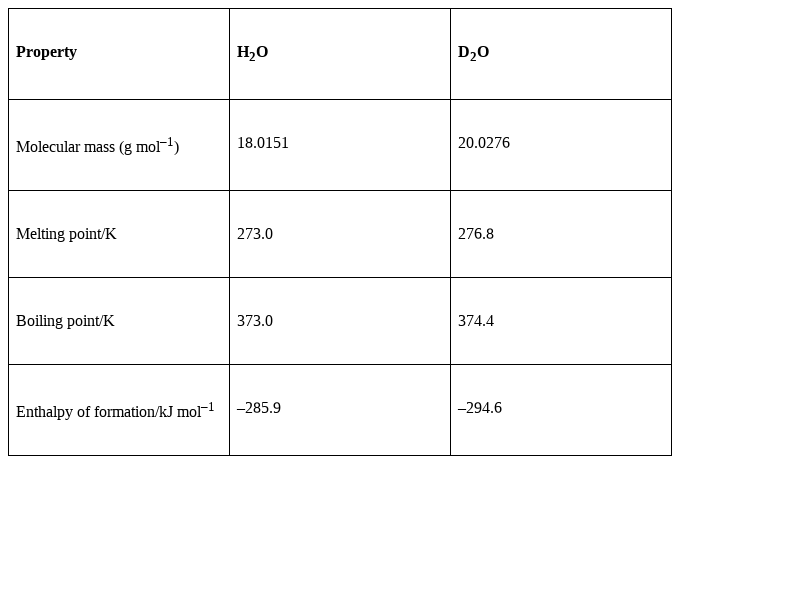
Reactions of D2O showing the exchange of hydrogen with deuterium are
HCl + D2O → HOD + DCl
NaOH + D2O → NaOD + HOD
NH4Cl + D2O → NH3DCl + HOD
1