Which of the two structures (A) and (B) given below is more stabilised by resonance? Explain.
CH3COOH and CH3COO-
(A) (B)
If we draw the resonating structure of the two compounds we have:
A: 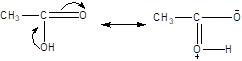
B: 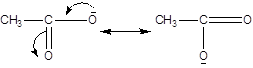
We know, that the compounds having more equivalent structure are more stable.
Now, the resonance in compound A, leads to generation of the positive charge on the Oxygen atom, whereas no such generation of the positive charge is found in the acetate ion i.e compound B.
Hence, the two resonating structure in compound A, are not equivalent, whereas the compound B, having negative charge on the oxygen atom is having the two equivalent structures, hence the compound B is more stable.
1