Match the type of mixture of compounds in Column I with the technique of separation/purification given in Column II.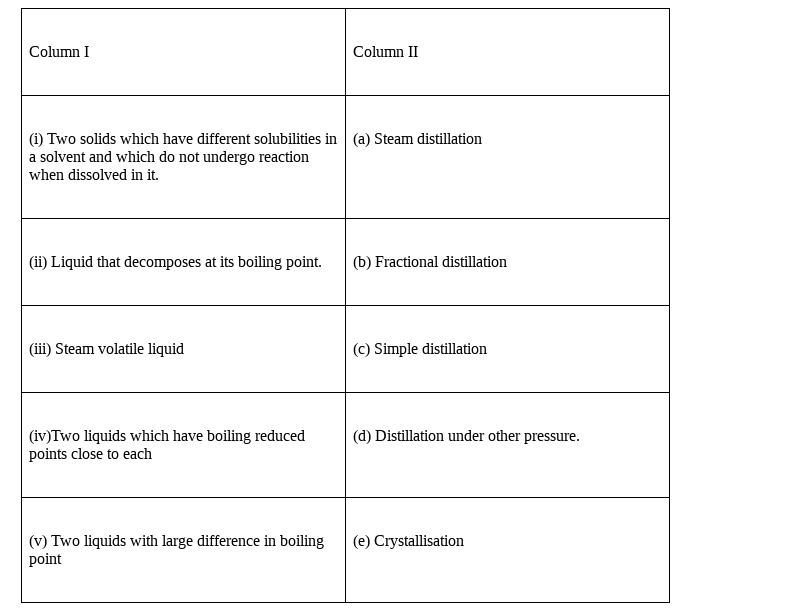
(i) – (e), (ii) – (d), (iii) – (a), (iv) – (b), (v) – (c)
Explanation:
(i) Two solids which have different solubility in a solvent and which do not undergo reaction when dissolved in it, is the condition for crystallisation. It is same as when the impurity and the compounds are dissolved they do not interact with each other.
Hence, option (e) is correct match.
(ii)Liquid that decomposes at its boiling point are affected by the temperature and hence can only be separated by the distillation method, as during distillation the compound is not directly heated but is heated by putting the flask in either oil bath or water bath.
Hence, option (d) is correct match.
(iii) Steam volatile liquid are separated by stem distillation as they are temperature sensitive materials and they are separated by steam distillation.
Hence, option (a) is correct match.
(iv)When the two liquids which have boiling points close to each other than the separation technique used is fractional distillation. As fractional distillation setup is made in such a way that the two liquid having close boiling point can also be separated very easily.
Hence, option (b) is correct match.
(v) Two liquids with large difference in boiling points, than simple distillation is best which can separate the two liquids. The setup is easy and affordable.
Hence, option (c) is correct match.