Match the terms mentioned in Column I with the terms in Column II.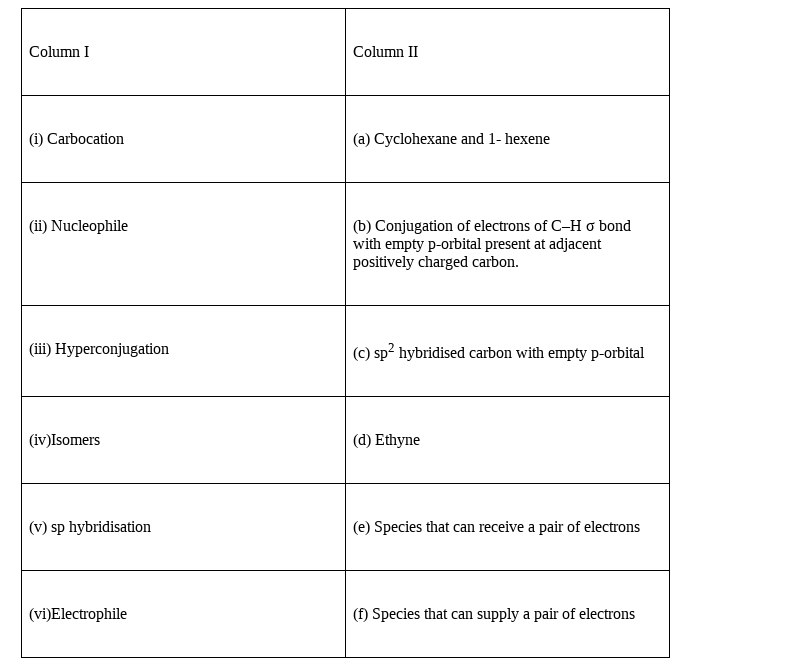
(i) – (c), (ii) – (f), (iii) – (b), (iv) – (a), (v) – (d), (vi) – (e)
Explanation:
(i)Carbocation is a species which the carbon atom has formed three sigma bonds and it has an empty p-orbital, hence option (c) is correct match.
(ii) We know nucleophile is a species which have electrons for the supply to the species which is able to accept, hence option (f) is correct match.
(iii) Hyperconjugation is defined as the phenomenon of Conjugation of electrons of C–H σ bond with empty p-orbital present at adjacent positively charged carbon, hence option (b) is correct match.
(iv) Isomers are those two compounds which are having same molecular formula but has different arrangement of atoms in the molecule.
The cyclohexane and 1-hexene are “ring-chain isomers” hence, option (a) is correct match.
(v) The molecule ethyne is having two C-atoms which are forming three bonds in between them.
And as out of the three bonds two are pi-bonds and only one is sigma bond, and also the sigma bond is formed with H. Hence, we find each C-atom forms only two sigma bond, we know for two sigma bonds the hybridisation is sp, hence, option (d) is correct match.
(vi)We know that the electrophile is the species which can accepts the electrons, as the word electrophile itself means the electron loving species, hence, option (e) is correct match.