Match the intermediates given in Column I with their probable structure in Column II.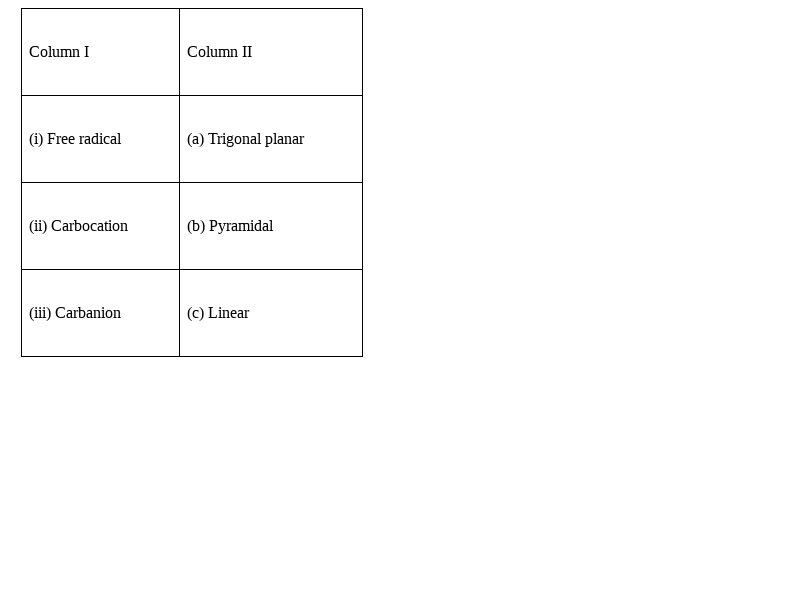
(i) – (a), (ii) – (a), (iii) – (b)
Explanation:
Free radical and the carbocation, both have the trigonal planar geometry, this is because, the hybridisation of the carbocation and the free radical is sp2 as they are having only two p-orbitals involved in the hybridisation along with s-orbital, and one p-orbital remains vacant.
Whereas the carbanion is having an extra electron, due to which it is an anion, and the extra electron is making the hybridisation sp3, and geometry is tetrahedral. Out of position in tetrahedral geometry one position is occupied by the electron pair, hence the the shape of the carbanion becomes pyramidal.
Hence, the match will be (i) – (a), (ii) – (a), (iii) – (b)