Write Lewis structure of the following compounds and show formal charge on each atom.
HNO3, NO2, H2SO4
Formal charge = valence electrons- (lone pair electrons + 1/2 bounded electrons)
(i) Formal charge calculations:
For N: 5 – 1/2 (8) = +1
For O: 6 – 6 – 1/2 (2) = -1
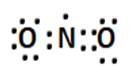
(ii) Formal charge calculations:
For N: 5 – 1/2 (8) = +1
For O without H: 6 - 6 - (1/2)2 = -1
For O without H on left side of N:6-4-1/2 (4)= 0
For O with H: 6 - 4 - (1/2)4 = 0
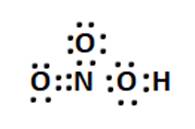
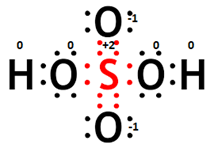
(iii) Formal charge calculations:
For S: 6 - (1/2)8 = +2,
For O without H: 6 - 6 - (1/2)2 = -1,
For O with H: 6 - 4 - (1/2)4 = 0.
1