The gyro-magnetic ratio of an electron in an H-atom, according to Bohr model, is
gyro-magnetic ratio of a particle is given by ![]() .
.
And angular moment of electron = ![]() where,
where,
n is the quantum number;
h is Planck’s constant;
Magnetic moment of electron in H-atom = ![]() where,
where,
n is the quantum number;
h is Planck’s constant;
e is charge of electron;
m is mass of electron.
So, gyro-magnetic ratio of a particle is given by 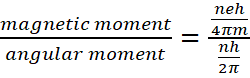
⇒ 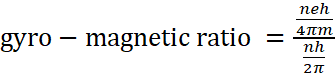
⇒ ![]() …(i)
…(i)
⇒ gyro-magnetic ratio of electron in h-atom is constant.
So, from (i) we can say that gyro-magnetic ratio of an electron in H-atom according Bohr-model is independent of the quantum number n and the orbit in which it is. And, will be negative as the charge of electron is a negative value.
Hence our answers are option (a) and (b).
1