Potassium chlorate decomposes, on heating, to form potassium chloride and oxygen. When 24.5 g of potassium chlorate is decomposed completely, then 14.9 g of potassium chloride is formed. Calculate the mass of oxygen formed. Which law of chemical combination have you used in solving this problem?
According to the above question-
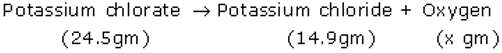
According to the law of conservation of mass-
Mass of potassium chloride + mass of oxygen = Mass of potassium chlorate
Mass of oxygen(x) = Mass of potassium chlorate- mass of potassium chloride
X = 24.5g-14.9g
X = 9.6g
Hence mass of oxygen = 9.6 g
76