In an experiment, 4.90 g of copper oxide was obtained form 3.92 g of copper. In another experiment, 4.55 g of copper oxide gave, on reduction, 3.64 g of copper. Show with the help of calculations that these figures verify the law of constant proportions.
According to question-
Reaction 1-
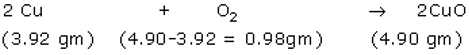
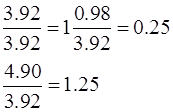
So, 1 equivalent of Cu reacts with 0.25 equivalent of O2 to form 1.25 equivalent of copper oxide.
Reaction 2-
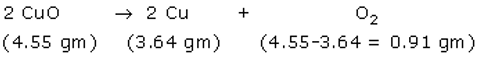
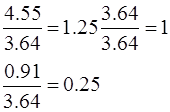
Here again, one can see that 1.25 equivalent of CuO decomposed to form 1 equivalent of Cu and 0.25 equivalent of oxygen.
Hence, law of constant proportion is verified.
77