Magnesium and oxygen combine in the ratio of 3 : 2 by mass to form magnesium oxide. What mass of oxygen gas would be required to react completely with 24 h of magnesium?
According to question-
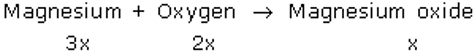
i.e. three equivalents of Mg reacts with 2 equivalents of O2 to form 1 equivalent of MgO.
When mass of Mg = 3x = 24 gm
So, x = 8 gm
Then, mass of oxygen required = 2x = 16 gm
78