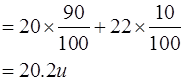Given that the percentage abundance of the isotope is 90% and that of the isotope is 10%, calculate the average atomic mass of neon.
Atomic mass = ∑Mass no. × % of that isotope
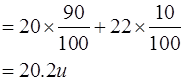
28
Given that the percentage abundance of the isotope is 90% and that of the isotope is 10%, calculate the average atomic mass of neon.
Atomic mass = ∑Mass no. × % of that isotope