An element Z contains two naturally occurring isotopes ![]() . If the average atomic mass of this element be 35.5 u, calculate the percentage of two isotopes.
. If the average atomic mass of this element be 35.5 u, calculate the percentage of two isotopes.
Given, Average atomic mass = 35.5 u
Let % amount of 35Z17 be y, then amount of 37Z17 is (100 - y).
So,
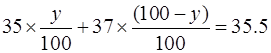
So, 35y + 3700 – 37y = 3550
Hence, y = 75
Thus, amount of 35Z17 is 75% and amount of 37Z17 is 25%
34