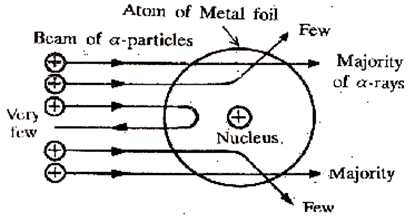
Rutherford’s α–particle scattering experiment showed that
(i) electrons have a negative charge
(ii) the mass and positive charge of the atom is concentrated in the nucleus
(iii) neutron exists in the nucleus
(iv) most of the space in an atom is empty
Which of the above statements are correct?
Rutherford’s ![]() - scattering experiment concluded that mass and positive charge of an atom is concentrated in the nucleus. This is because very few alpha particles were deflected by 180 degrees from their path. Most of the alpha particles passed straight through the gold without getting deflected. This indicates that most of the space in an atom is empty.
- scattering experiment concluded that mass and positive charge of an atom is concentrated in the nucleus. This is because very few alpha particles were deflected by 180 degrees from their path. Most of the alpha particles passed straight through the gold without getting deflected. This indicates that most of the space in an atom is empty.
9