Complete the Table 4.1 on the basis of information available in the symbols given below
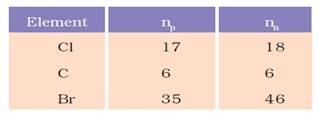
The mass number of chlorine is 35 and atomic number is 17.
Number of neutrons is equal to the mass number - number of protons.
It means it has 35-17= 18 Neutrons.
Similarly, in case of carbon atomic number is 6 and its mass number is 12. hence, the number of protons is 6 and the number of neutrons is also 6.
Bromine has atomic number 35 which means it has 35 protons and 46 neutrons.
33