Using electron-dot diagrams which show only the outermost shell electrons, show how a molecule of nitrogen, N2, is formed from two nitrogen atoms. What name is given to this type of bonding? (Atomic number of nitrogen is 7)
The number of electrons in the outer most shell of the nitrogen is 5 so, to achieve the 8-electron structure of an inert gas, it needs 3 more electrons and hence combines with another nitrogen atom to form a molecule of nitrogen gas.
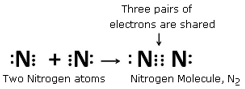
This type of bonding is called covalent bonding.
37