A metal which exists as a liquid at room temperature is obtained by heating its sulphide ore in the presence of air.
(a) Name the metal and write its chemical symbol.
(b) Write the name and formula of the sulphide ore.
(c) Give the equations of chemical reactions involved in the production of metal from its sulphide ore.
(d) Name a common 'device in which this metal is used.
(e) Can this metal displace copper from copper sulphate solution? Why?
(a) The name of the metal is mercury. And its chemical formula is Hg.
(b) The name and formula of the sulphide ore is Cinnabar and HgS.
(c) The equations of chemical reactions involved in the production of metal from its sulphide ore is
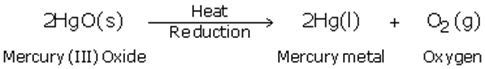
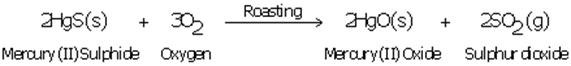
(d) Mercury is used in thermometer.
(e) Mercury cannot displace copper from copper sulphate solution because it is less reactive then copper.
93