(a) Give the names and structural formulae of the next two higher homologues of methane.
(b) The molecular formula of a hydrocarbon is C2H5. Name its homologues series.
(c) Select the hydrocarbons which are members of the same homologues series. Give the name of each series. C5H10; C3H8; C4H10; C7H12; C8H16
(a) The next two Higher homologues of methane are ethane and propane.
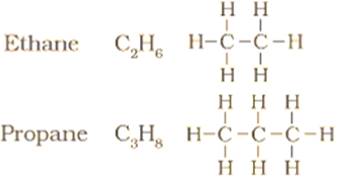
(c) Homologous series is a series of organic compounds having similar properties in which the successive members differ by a -CH2 group.
Alkanes: C3H8; C4H10
Alkenes: C5H10; C8H16
Alkynes: C7H12
27