If one of the two electrons of a H2 molecule is removed, we get a hydrogen molecular ion H+2. In the ground state of an H+2, the two protons are separated by roughly 1.5 Å, and the electron is roughly 1 Å from each proton. Determine the potential energy of the system. Specify your choice of the zero of potential energy.
The system of two protons and electron is represented as below,
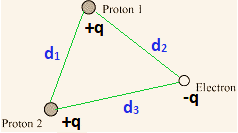
The total potential energy at infinity is Zero.
Thus, Potential energy of the system is,
V = ![]()
Where,
Charge on proton 1, q1 = 1.6 × 10-19 C
Charge on proton 2, q2 = 1.6 × 10-19 C
Charge on electron, q3 = - 1.6 × 10-19 C
Distance between proton 1 and proton 2, d1 = 1.5 × 10-10 m
Distance between proton 1 and electron, d2 = 1 × 10-10 m
Distance between proton 2 and electron, d3 = 1 × 10-10 m
ϵ0 = Permittivity of free space
and, ![]()
∴ V = 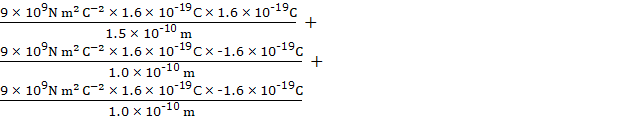
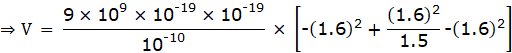
⇒ V = -30.7 × 10-19 J
Writing in electron volts, we get,
⇒ V = -19.2 eV
(∵ 1eV = 1.6 × 10-19 J)
Therefore, the potential energy of the system is -19.2 eV.