Calculate the
(a) momentum, and
(b) de Broglie wavelength of the electrons accelerated through a potential difference of 56 V.
(a) When an charged particle having charge q is accelerated by a potential difference V volts it gain kinetic energy given by
K = Vq, which imparts it with velocity
We know kinetic energy of a Particle with mass m kg and velocity v is given by
![]()
Equating both equations
![]()
We get velocity of the particle as
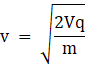
But we know momentum is given by relation
P = mv
Where P is the momentum of particle having mass m and moving with velocity v
So we get
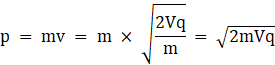
Here Potential difference
V = 56 volts
q = 1.6 × 10-19 C
m = 9.1 × 10-31Kg
so momentum of the electron,
![]()
![]()
So momentum of electron is 4.04 × 10-24 Kgms-1
(b) Now we know de Broglie wavelength of a Particle is given by relation