When ethanoic acid reacts with sodium hydrogencarbonate, then a salt X is formed and a gas Y is evolved. Name the salt X and gas Y. Describe an activity with the help of a labelled diagram of the apparatus used to prove that the evolved gas is the one which you have named. Also write the chemical equation of the reaction involved.
Salt X is sodium ethanoate, CH3COONa; Gas Y is carbon dioxide (CO2)
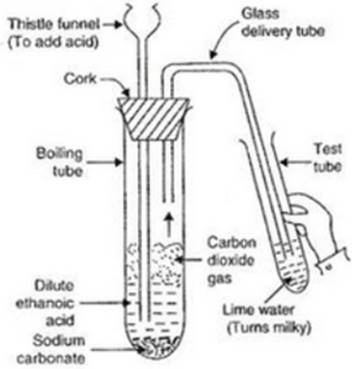
Activity:
� Take a boiling test tubes and put about 0.5 g of sodium carbonate in it.
� Also, take another tube and add some lime water in it.
� Then add 2 ml of dilute ethanoic acid in the boiling tube A. We will observe that brisk effervescence of carbon dioxide is produce.
� Pass the gas produced through lime water. Lime water turns milky.
� So, this experiment proves that when ethanoic acid reacts with sodium carbonate, then carbon dioxide gas is produced.
![]()
46