An organic acid X is a liquid, which often freezes during winter time in cold countries, having the molecular formula C2H4O2. On warming it with methanol in the presence of a few drops of concentrated sulphuric acid, a compound Y with a sweet smell is formed.
(a) Identify X and Y. Also write their formulae showing the functional group present in them.
(b) Write a chemical equation for the reaction involved.
(a) X is ethanoic acid.
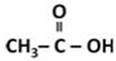
Y is methyl ethanoate.
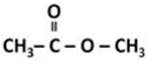
![]()
71