With the help of labeled diagrams, describes an activity to show that acids produce ions only in aqueous solutions.
(ii) Add some concentrated sulphuric acid to the test tube.
(iii) Fit a rubber cork with a small delivery tube in the mouth of the test tube. Concentrated sulphuric acid reacts with sodium chloride to form hydrogen chloride gas. The hydrogen chloride gas starts coming out of the open end of the glass tube.
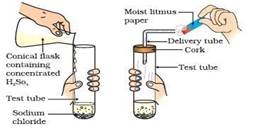
Observations- we will test the gas evolved successively holding a blue and red litmus paper above test tube containing HCl gas. There is no change in colour of the blue litmus paper. This shows that HCl gas does not behave as an acid in the absence of water. However, when we hold blue litmus paper in HCl gas, we will see that the blue litmus paper turns red.
Conclusion- The above experiment suggests that hydrogen ions in HCl are produced in the presence of water. The separation of H+ ion from HCl molecules cannot occur in the absence of water. HCl + H2O → H3O+ + Cl-
Hydrogen ions cannot exist alone, but they exist after combining with water molecules. Thus hydrogen ions must always be shown as H+(aq) or hydronium ion (H3O+). H+ + H2O → H3O+ This indicates that HCl gas shows acidic behavior in the presence of water as hydrogen ions are formed.