Compounds such as alcohol and glucose also contain hydrogen but are not categorized as acids. Describe an activity to prove it.
Experiment- Two nails are fitted on a cork and are kept in a 100 mL beaker. The nails are then connected to the two terminals of a 6-volt battery through a bulb and a switch. Now pour some dilute HCl in the beaker and the current is switched on. The same experiment is then performed with glucose solution and alcohol solution.
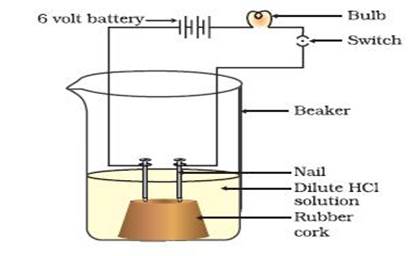
Observations- It will be observed that the bulb glows in the HCl solution and does not glow in the glucose solution and alcohol solution. Result- HCl dissociates into H+and Cl−ions. These ions conduct electricity in the solution resulting in the glowing of the bulb. On the other hand, the glucose and alcohol solution does not dissociate into ions. Therefore, it does not conduct electricity.
Conclusion- From this activity, it can be concluded that all acids contain hydrogen but not all compounds containing hydrogen are acids. That is why, although alcohols and glucose contain hydrogen, they are not categorised as acids.
33