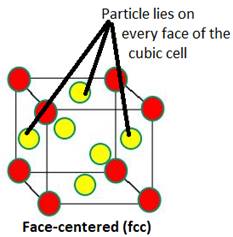
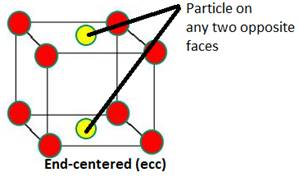
Distinguish between
Face-centred and end-centred unit cells.
Face-centered | End- centered |
An fcc refers to a crystal structure consisting of an atom at each cube corner and an atom in the center of each cube face. | An ecc refers to a crystal structure consisting of an atom at each cube corner and an atom in the center of any two opposite faces. |
|
|
Examples: tungsten, chromium. | Examples: Gold, silver, copper, nickel, etc. |
14