If the radius of the octahedral void is r and radius of the atoms in close packing is R, derive relation between r and R.
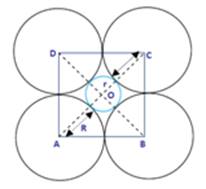
In the given figure, let the sphere have the centre, O and is fitted in the octahedral void.
As given, radius of the sphere fitted in the octahedral void = r
And the radius of the atoms in close packing = R
Here, angle AOD = 900
In triangle AOD,
DA2 = OA2 + OD2
⇒ (R+R)2 = ( R + r )2+ (R+r)2
⇒ 4R2 = 2(R+r)2
⇒ 2R2 = (R+r)2
⇒ √2 R = (R+r)
⇒ R + r = ![]() R
R
⇒ r = ![]() R – R
R – R
⇒ r = R (1.414 - 1)
⇒ r = 0.414 R
This is the required relation between r and R.
15