Calculate the mass percentage of benzene (C6H6) and carbon tetrachloride(CCl4) if 22 g of benzene is dissolved in 122 g of carbon tetrachloride.
Given,
Benzene (C6H6) is 22g in the solution.
Carbon tetrachloride (CCl4) is 122g in solution
We know,
Mass Percentage of solute = ![]()
Total mass of the solution = 22g (benzene) + 122g (carbon tetrachloride)
= 144g
Mass percentage of benzene (C6H6) ![]()
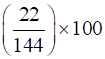 = 15.28%
= 15.28%
Note: The second method to calculate the Mass percentage of carbon tetrachloride = 100 – Mass % of C6H6
= 100 - 15.28%
= 84.72%
91