H2S, a toxic gas with rotten egg like smell, is used for the qualitative analysis. If the solubility of H2S in water at STP is 0.195 m, calculate Henry’s law constant.
According to Henry's law,
"At a constant temperature, the amount of a given gas that dissolves in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid."
Stated as,
p = KHx
Where,
P = partial pressure of the solute above the solution
KH = Henry's constant
x = concentration of the solute in the solution
Given,
Solubility of H2S in water at STP is 0.195 m
We know,
At STP pressure p = 0.987 bar.
0.195 mol of H2S is dissolved in 1000g of water
Moles of water = 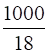
= 55.56 g/mol
∴ the mole fraction of H2S = 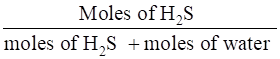
= 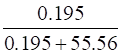
= 0.0035
According to Henry's law,
![]() p = KHx
p = KHx
![]() KH =
KH = 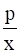
![]() KH =
KH = 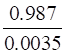
![]() KH = 282 bar
KH = 282 bar
∴ The Henry’s law constant is 282 bar