The vapour pressure of pure liquids A and B are 450 and 700 mm Hg respectively, at 350 K. Find out the composition of the liquid mixture if total vapour pressure is 600 mm Hg. Also find the composition of the vapour phase.
Given,
PAo = 450 mm Hg
PBo = 700 mm Hg
ptotal = 600 mm of Hg
By using Rault's law,
ptotal = PA + PB
ptotal = PAoxA + PBoxB
ptotal = PAoxA + PBo( 1 - xA )
ptotal = (PAo- PBo)xA + PBo
600 = (450 - 700) xA + 700
-100 = -250 xA
xA = 0.4
∴ xB = 1 - xA
xB = 1 – 0.4
xB = 0.6
Now,
PA = PAoxA
PA = 450 × 0.4
PA = 180 mm of Hg
and
PB = PBox
PB = 700 × 0.6
PB = 420 mm of Hg
Composition in vapour phase is calculated by
Mole fraction of liquid, 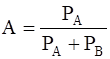
= 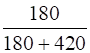
= 0.30
Mole fraction of liquid, 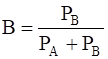
= 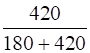
= 0.70
Note: Alternate method to find the Mole fraction of liquid B is
= 1 - Mole fraction of liquid A
= 1 – 0.30
= 0.70