Calculate the osmotic pressure in pascals exerted by a solution prepared by dissolving 1.0 g of polymer of molar mass 185,000 in 450 mL of water at 37°C.
Given,
Volume of water, V = 450 mL = 0.45 L
Temperature, T ![]() (37 + 273)K = 310 K
(37 + 273)K = 310 K
1.0 g of polymer of molar mass 185,000
Number of moles of polymer, n = 1 / 185,000 mol
We know that,
Osmotic pressure, π = 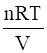
= 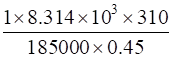
= 30.98 Pa
= 31 Pa (approx)
23