What role does the molecular interaction play in a solution of alcohol and water?
The lower members of alcohols are completely miscible [highly soluble] with water but the solubility decreases with increase in the molecular weight. The lower members of the alcohol group have the capability to form intermolecular hydrogen bonding with water molecules as alcohols are polar molecules in nature.
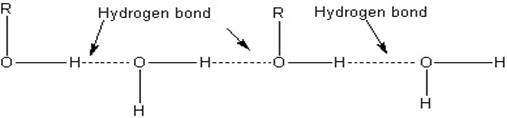
Alkyl groups are hydrophobic [prevents formation of hydrogen bonds with water] in nature. In lower alcohols, the alkyl group is small and the –OH group of alcohol is effective in making hydrogen bonds with water.
But with the increase in the size of alkyl group, the hydrophobic [water hating] nature of alkyl group dominates over the hydrophilic [water liking] nature of –OH group making the molecule less soluble in water. So solubility of alcohols decreases with increase in its molecular mass.
Since molecular interaction is weaker between higher alcohols and water, as a result, when alcohol and water are mixed, the intermolecular interactions become weaker and the molecules can easily get liberated off. This increases the vapour pressure of the solution, which in turn lowers the boiling point of the resulting solution.