What is meant by positive and negative deviations from Raoult's law and how is the sign of Δmix related to positive and negative deviations from Raoult's law?
Raoult’s law states that at a given temperature, the vapour pressure of a solution containing non volatile solute is directly proportional the mole fraction of the solvent.
Non ideal solutions show positive and negative deviations from ideal behaviour.
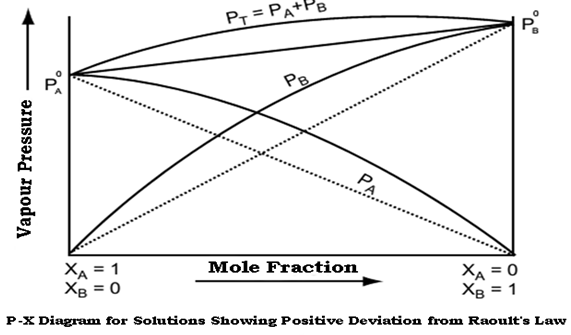
Non ideal solutions showing positive deviations from Raoult’s law-
A solution is said to show positive deviation from Raoult’s Law when at any composition, its vapour pressure is higher than that given by Raoult’s Law.
The positive deviation is shown by those liquid pairs in which the A-B molecular interaction forces are weaker than the corresponding A-A or B-B molecular interaction forces. When the A-B molecular interaction forces are weaker, then molecules of liquid A find it easier to escape as compared to pure solution thereby increasing the vapour pressure of solution.
As a result, each component of solution has a partial vapour pressure greater than expected on the basis of Raoult’s law. This is called positive deviations from Raoult’s law, that is PA>PAoXA and PB>PBoXB
∆mixH [Change in Enthalpy] is positive because energy is required to break A-A & B-B attractive forces. Hence it is an endothermic process.
Examples of liquid Pairs:
1. Water + methanol
2. Water + ethanol
3. Acetone + benzene
4. Carbon tetrachloride + benzene
The graph of vapour pressure and mole fraction for liquids showing positive deviation from Raoult’s Law is shown below:
From the diagram it is clear that the composition curve of such a solution lies above the composition curve obtained on basis of Raoult’s Law.
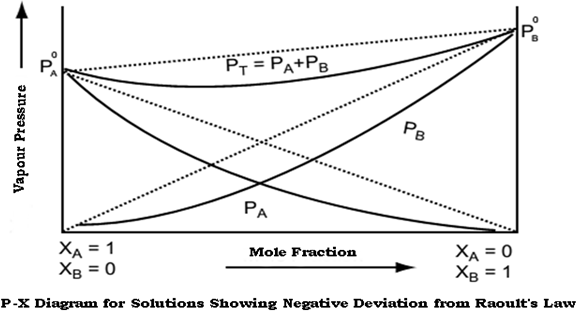
Non ideal solutions showing Negative deviations from Raoult’s law –
When the vapour pressure of the solution is lower than that calculated on the basis of Raoult’s Law, the solution is said to show negative deviation from Raoult’s Law. Even the vapour pressure of individual components show negative deviation from Raoult’s Law
The negative deviation is shown by those liquid pairs in which the A-B molecular interaction forces are stronger than the corresponding A-A or B-B molecular interaction forces. When the A-B molecular interaction forces are stronger, then molecules of liquid A find it easier to escape from pure solution as compared to the mixture thereby decreasing the vapour pressure of solution.
Consequently, each component of solution has a partial vapour pressure less than expected on the basis of Raoult’s law. This is called negative deviations from Raoult’s law, i.e. PA<PAoXA & PBoXB.
Examples of liquid pairs:
1. Water + hydrochloric acid
2. Water + nitric acid
3. Chloroform + benzene
4. Acetone + aniline
∆mixH is negative because energy is released due to increase in attractive forces. Hence it is an exothermic process.
The graph of vapour pressure and mole fraction for liquids showing negative deviation from Raoult’s Law is shown below: