Benzene and naphthalene form ideal solution over the entire range of composition. The vapour pressure of pure benzene and naphthalene at 300 K are 50.71 mm Hg and 32.06 mm Hg respectively. Calculate the mole fraction of benzene in vapour phase if 80 g of benzene is mixed with 100 g of naphthalene.
Given-
![]() of benzene = 50.71 mm Hg
of benzene = 50.71 mm Hg
![]() of naphthalene = 32.06 mm Hg
of naphthalene = 32.06 mm Hg
Using the formula of lowering vapour pressure to calculate mole fraction of benzene and naphthalene in solution,
![]()
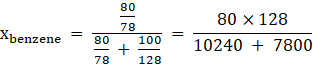
![]()
Thus, ![]()
![]()
Using formula for vapour pressure,
![]()
![]()
![]()
![]()
![]()
Mole fraction of benzene in vapour phase is calculated by,
![]()
![]()
![]()
Thus, mole fraction of benzene in vapour phase is 0.6744
43