The air is a mixture of a number of gases. The major components are oxygen and nitrogen with approximate proportion of 20% is to 79% by volume at 298 K. The water is in equilibrium with air at a pressure of 10 atm. At 298 K if the Henry’s law constants for oxygen and nitrogen at 298 K are 3.30 × 107 mm and 6.51 × 107 mm respectively, calculate the composition of these gases in water.
Given-
KH for O2 = 3.30 × 107 mm Hg,
KH for N2 = 6.51 × 107 mm Hg
Percentage of oxygen (O2) = 20 %
Percentage of nitrogen (N2) = 79%
Total pressure = 10 atm
Using Henry’s law,
→![]()
![]()
where, p is the partial pressure of gas in the solution and KH is Henry’s constant.
Now, to determine the mole fraction of oxygen in solution, ![]() , we use
, we use
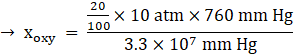
![]()
To determine the mole fraction of nitrogen in solution, ![]() ,
,
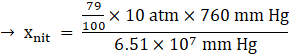
![]()
Thus, the mole fraction of oxygen in solution, xoxy = 4.61x10-5
and the mole fraction of nitrogen in solution, xnit is 9.22x10-5