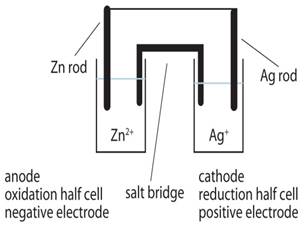
Depict the galvanic cell in which the reaction
Zn(s) + 2Ag+ (aq) → Zn2+ (aq) + 2Ag(s) takes place. Further show:
1) Which of the electrode is negatively charged?
2) The carriers of the current in the cell.
3) Individual reaction at each electrode.
The galvanic cell corresponding to the given redox reaction can be represented as:
Zn|Zn2 + (aq)||Ag + (aq)|Ag
1) Zn electrode (anode) is negatively charged because, at this electrode, Zn is oxidized to Zn2+, causing electron accumulation at the anode.
2) Electrons (ions) are the carriers of the current in the cell and in the external circuit, current flows from Ag (cathode) to Zn(anode) which is normally opposite to the electron flow which is from anode to cathode.
3) At anode:
Zn(s)⇒ Zn2 + (aq) + 2e–
At cathode:
Ag + (aq) + e –⇒ Ag(s)