The rate constant for a first order reaction is 60 s–1. How much time will it take to reduce the initial concentration of the reactant to its 1/16th value?
Given:
Order of the reaction = 1
Let, Initial concentration [R]°= x
Final concentration [R] = x/16
Rate constant k = 60 s-1
We know, time ![]()
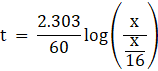
⇒ 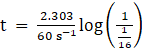
⇒ ![]()
Solving, we get t = 4.6 × 10-2s
18