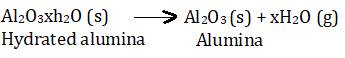How can you separate alumina from silica in a bauxite ore associated with silica? Give equations, if any.
To separate alumina from silica in bauxite ore associated with silica, firstly, the powdered ore is digested with a concentrated NaOH solution at a temperature of 473 – 523K and 35 – 36 bar pressure. This results in the leaching out of alumina (Al2O3) as sodium aluminate and silica (SiO2) as sodium silicate leaving the impurities behind.
Al2O3 (s) + 2NaOH (aq) + 3H2O (l) → 2 Na[Al(OH)4] (aq)
Alumina
SiO2 + 2NaOH (aq)→ Na2SiO3 (aq) + H2O (l)
Silica
After this, CO2 gas is passed through the resultant solution to neutralize the alumina present in the solution, this results in the precipitation of hydrated samples of alumina. To include precipitation, the solution is seeded with freshly prepared samples of hydrated alumina.
![]()
During this process, sodium silicate remains in the solution. The hydrated alumina obtained is then filtered, dried, and heated to get back pure alumina.