Name the processes from which chlorine is obtained as a by-product. What will happen if an aqueous solution of NaCl is subjected to electrolysis?
In the electrolysis of NaCl by Down’s process, chlorine is obtained as a by-product. This process involves the electrolysis of a fused mixture of NaCl and CaCl2 at 873K. during electrolysis, sodium is liberated at the cathode and Cl2 is liberated at the anode.
![]()
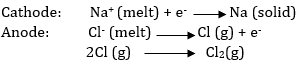
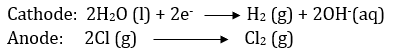
If an aqueous solution of NaCl is electrolysed, H2 is evolved at the cathode and Cl2 is obtained at the anode. the reason being that E0 of Na+/Na redox couple is much lower (E0 = - 2.71 V) than that of H2O (E0H2O/H2) = - 0.83V ) and hence water is reduced to H2 in presence of Na+ ions. However, NaOH is obtained in the solution.
![]()
24