Outline the principles of refining of metals by the following methods:
(i) Zone refining
(ii) Electrolytic refining
(iii) Vapour phase refining
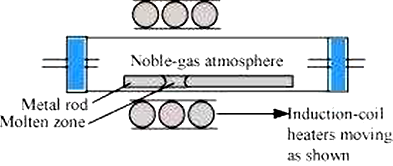
(i) Zone refining Is the method based on the principle that the impurities are more soluble in the melt than in the solid state of the metal.
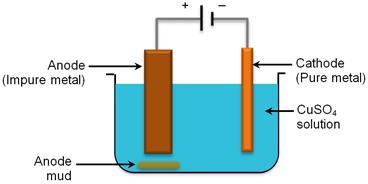
(ii) Electrolytic refining works on the principle of refining impure metals by the use of electricity. In Electrolytic refining, the impure metal is made the anode and a strip of pure metal is made as the cathode. A solution of a soluble salt of the same metal is taken as the electrolyte. When an electric current is passed, metal ions from the electrolyte are deposited at the cathode as a pure metal and the impure metal from the anode dissolves into the electrolyte in the form of ions. The impurities present in the impure metal gets collected below the anode.
This is called as the anode mud.
![]()
(iii) Vapour refining is the process of refining metal by converting it into its volatile compound and then, decomposing it to obtain a pure metal. To carry out this process,
i) the metal should form a volatile compound with an available reagent, and
ii) The volatile compound should be easily decomposable so that the metal can be easily recovered.
iii) Nickel, Zircinium. And titanium are refined using this method.