[Fe(H2O)6]3+ is strongly paramagnetic whereas [Fe(CN)6]3-is weakly paramagnetic. Explain.
In [Fe(H2O)6]3+
Electronic configuration of Fe is: [Ar]3d64s2
[Ar] = 1s22s22p63s23p6
Electronic configuration of Fe+3 = [Ar]3d5
Outer electronic configuration of Fe+3 = 3d5
Overall charge balance:
X + 6(0) = 3
X = + 3
In [Fe(CN)6]3-
Overall charge balance:
X + 6(-1) = -3
X = + 3
In both the compounds Fe is in + 3 oxidation state.
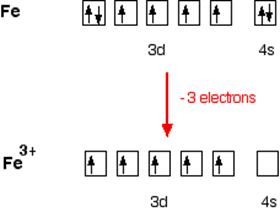
In case of [Fe(H2O)6]3+
H2O is weak field ligand so it does not pair the unpaired electron. Total no. of the unpaired electron, n = 5.
Spin only magnetic moment is given by:
μ = [n(n + 2)]1/2
μ = [5×7]1/2
μ = 5.916BM
In case of [Fe(CN)6]3-
CN- is a strong field ligand so it pairs up the electron.
Total no. of unpaired electrons = 1
Spin only magnetic moment is given by:
μ = [n(n + 2)]1/2
μ = [1×3]1/2
μ = 1.732BM
as we can see spin only magnetic moment of [Fe(H2O)6]3+ is more than [Fe(CN)6]3- .
so, [Fe(H2O)6]3+ is strongly paramagnetic whereas [Fe(CN)6]3 weakly paramagnetic.
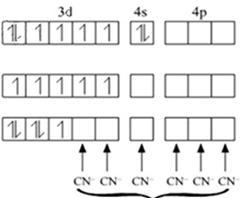
Fe in ground state
Fe in + 3 oxidation state
Electrons from 5 CN- ligands