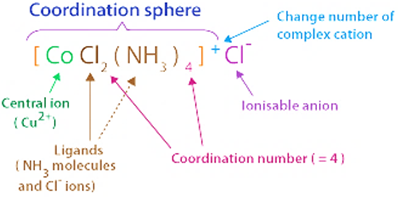
Explain the bonding in coordination compounds in terms of Werner’s postulates.
Bonding in coordination compounds in terms of Werner’s postulates is explained as:
a) Metals can show two types of valencies which are Primary valency and Secondary valency.
1. Primary Valency: Primary Valency shows Oxidation state. Primary valencies are ionizable.
2. Secondary Valency: Secondary Valency shows coordination number. These are non-ionizable.
b) Both Primary and secondary valency of the metal are to be satisfied which is done by negative ions in case of primary valency and negative or neutral species in case of secondary valency.
c) Metals have a fixed number of secondary valencies/ Coordination number around the central atom, these secondary valencies are placed in such a way which leads to a specific geometry of the coordination compound.