Explain with two examples each of the following: coordination entity, ligand, coordination number, coordination polyhedron, homoleptic and heteroleptic.
1) Coordination Entity: Coordination entity is a charged entity having positive or negative charge in which the central atom is surrounded by molecules which may be neutral/negatively charged called Ligands.
Examples:
i. Cationic Complexes: [Cu(H2O)6]2+ , [Al(H2O)6]3+
ii. Anionic Complexes: [CuCl4]2- , [Al(H2O)2(OH)4]-
iii. Neutral Complexes: [Co(NH3)4 Cl2] ,[Ni(CO)4]
2) Ligands: Ligands are the neutral or negatively charged entities surrounding the central metal atom of the coordination complex which possesses at least one unshared pair of electrons
Example: F-, Cl-, Br-, I-, H20, and NH3
3) Coordination Number: Coordination Number which is also called as Ligancy is the total number of ligands that are attached to the central metal atom of the coordination complex.
Example: [Cr(NH3)2Cl2Br2]− has Cr3+ as its central cation, and has a coordination number of 6
Al3+ has coordination number 4 in [AlCl4]- but 6 in [AlF6]3-.
4) Coordination polyhedron: Coordination polyhedron is defined as the spatial arrangement of the ligands around the central atom of a coordination complex.
Example:
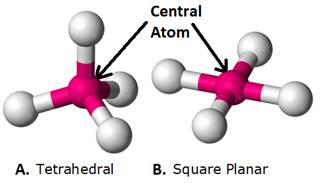
In the figure: The pink Sphere depicts the central atom of the coordination entity.
5) Homoleptic complexes: Complexes in which the central metal atom is surrounded only by the same kind of donor groups which are the ligands.
Example: [Ag(CN)2]-,[Fe(CN6)]4+ etc
6) Heteroleptic complexes: Complexes in which the central metal atom is surrounded by more than one kind of donor groups which are the ligands.
Example: [Co(NH3)4 Cl2] + ,[Co(NH3)5 Cl]2+