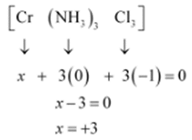Specify the oxidation numbers of the metals in the following coordination entities:
(i) [Co(H2O)(CN)(en)2]2+
(ii) [CoBr2(en)2]+
(iii) [PtCl4]2–
(iv) K3[Fe(CN)6]
(v) [Cr(NH3)3Cl3]
(i) Let Oxidation no. of Co be x and charge on the complex is given as + 2
H2O has Oxidation Number: 0
CN has Oxidation Number: -1
en has Oxidation Number :0
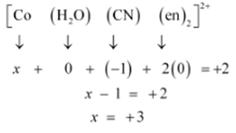
(ii) Let Oxidation number of Co be x and charge on the complex is given as + 1
Br has Oxidation number: 1
en has oxidation number : 0
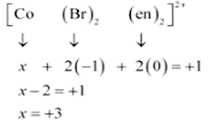
(iii) Let Oxidation number of Pt be x and charge on the complex is given as -2
Cl has oxidation number : -1
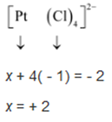
(iv) This complex can also be seen as [Fe(CN)6]3-
Let Oxidation number of Fe be x and charge given on the complex is given as -3
CN has oxidation number : -1
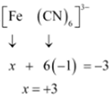
(v) Let Oxidation number of Cr be x and charge given on the complex is given as 0
NH3 has oxidation number : 0
Cl has oxidation number: -1