List various types of isomerism possible for coordination compounds, giving an example of each.
Isomers are the compounds which have same chemical formula but different arrangement of atoms in space. There are principle two types of isomerism:
(i) Stereo isomerism:
(a) geometrical isomerism
(b) optical isomerism
(ii) structural isomerism
(a) Ionisation isomerism
(b) Linkage isomerism
(c) Coordination isomerism
(d) Solvate isomerism
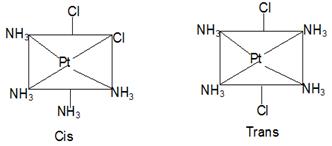
Geometrical isomerism comes into existence by the different spatial arrangement of groups around the central metal atom. Similar groups may either be arranged on the same side or on opposite sides of the central metal atom. This gives rise to two types of isomers called cis and trans isomers. When groups under consideration are arranged on the same side of the central metal atom, isomers are called cis isomers and when the groups under consideration are spatially placed on the opposite sides, isomers are called trans isomer.
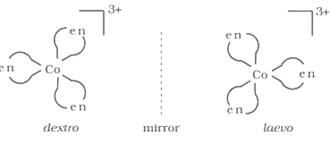
Optical isomerism is exhibited by those compounds which possess chirality. The presence of an element of symmetry makes a molecule symmetric and renders it optically inactive. When molecule does not possess any element of symmetry its mirror image is non superimposable with the molecule itself. This makes the molecule optically active. Such an asymmetric molecule can show the phenomenon of optical .The two forms of the molecule which are mirror images of each other are called enantiomers.One form rotates the plane of plane polarized light in clockwise direction, while the other in anticlockwise direction. The former is called the d-form, while the latter is termed as l-form.
Ionisation isomerism: In ionisation isomerism there is an exchange of ions inside and outside the coordination sphere. Ionisation isomers have the same formula but produce different ions in solution. It is also known as ion-ion exchange isomerism.
Examples:
ions present in the solution | Compounds | Colour |
[Co(NH3)5 SO4] + + Br- | [Co(NH3)5 SO4]Br | Red violet |
Co(NH3)5Br] + + + SO42- | Co(NH3)5Br]SO4 | Red |
In the two isomers (A) and (B), there is an exchange of ions inside and outside the coordination sphere. The aqueous solution of (A) gives the precipitate of AgBr on treatment with AgNO3 as it contains Br, while that of (B) gives precipitate of BaSO4, on treatment with BaCl2.
Linkage isomerism: In linkage isomerism the same ligand is bonded to central metal atom or ion through different atoms. Linkage isomers have the same molecular formula but differ in the linkage of the ligand to central metal atom.
For example, -NO, ligand can bind to the central metal through nitrogen or oxygen to give two isomers as shown below.
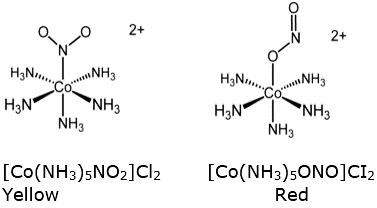
In isomer A nitro group is bonded to the central metal atom through nitrogen and is known as nitro while in the isomer B it is bonded through Oxygen and is known as nitrito.
Coordination isomerism: this type of isomerism arises from different Complex ions having same molecular formula. There is interchange of ligands between cationic and anionic entities of different metal ions present in the complex. Example is [Co(NH3)6][Cr(CN)6] in which ammonia ligands are bounded to Cobalt and cyanide ligands to chromium ion. In its coordination isomer [Cr(NH3)6][Co(CN)6] ammonia ligands are bounded to chromium ion and cyanide ligands to Cobalt ion.
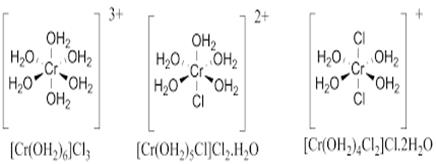
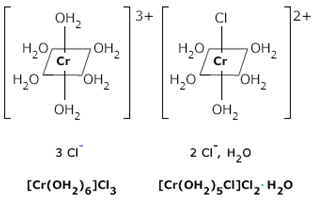
Solvent isomerism or hydrate isomerism: in hydrate isomerism there is an exchange of water molecule inside and outside coordination sphere. Hydrate isomers have the same molecular formula but differ in the number of molecules of water inside and outside the coordination sphere.

Ex. CrCl3.6H2O exists in 3 hydrate isomers:
Compound | Colour | Number of water molecules inside coordination sphere | Number of water molecules outside coordination sphere |
[Cr(H2O)6]Cl3 | Violet | 6 | 0 |
[Cr(H2O)5Cl]Cl2H2O. | Blue Christmas | 5 | 1 |
[Cr(H2O)4Cl2]Cl.H2O | Green | 4 | 2 |