How many geometrical isomers are possible in the following coordination entities?
(i) [Cr(C2O4)3]3– (ii) [Co(NH3)3Cl3]
(i) No geometrical isomer is possible for [Cr(C2O4)3]3– because the ligand C2O42- is bidentate ligand(which have two sites of attachment to the central atom) and also in the coordination sphere it is the only ligand bond to it.
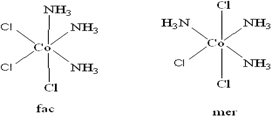
(ii) In the coordination sphere of [Co(NH3)3Cl3] there are two types of ligands present i.e NH3 and Cl- . Coordination number is 6. There are 2 isomers possible for the complex:
Facial: In this isomer one type of ligand say NH3 forms the face of the square bipyramidal (triangular) structure.
Meridional: In this isomer one type of ligand are along the central axis of the pyramidal structure.
13